BEEASY CONSULTING SRLS
VIALE F. STROZZI, 30 - 50129 FIRENZE (ITALY) * VAT n.: IT07244890484 * REA REGISTRATION n.: FI–689983 * FULLY PAID COMPANY CAPITAL: € 5.000,00

UNLOCK THE FULL POTENTIAL OF YOUR RESEARCH
BY REMOVING UNCERTAINTY
"By failing to prepare, you are preparing to fail."
Benjamin Franklin
IS THERE A SINGLE DEFINITION OF "FEASIBILITY"?

In the realm of clinical studies, thorough preparation and strategic foresight serve as the cornerstones of success. Central to this preparation is the vital process of feasibility assessment, which evaluates the practicality, resources, and structure within the scope of the entire project. Feasibility is the backbone of any successful project - it identifies hurdles, fine-tunes strategies, and transforms plans into actionable pathways for impactful results.
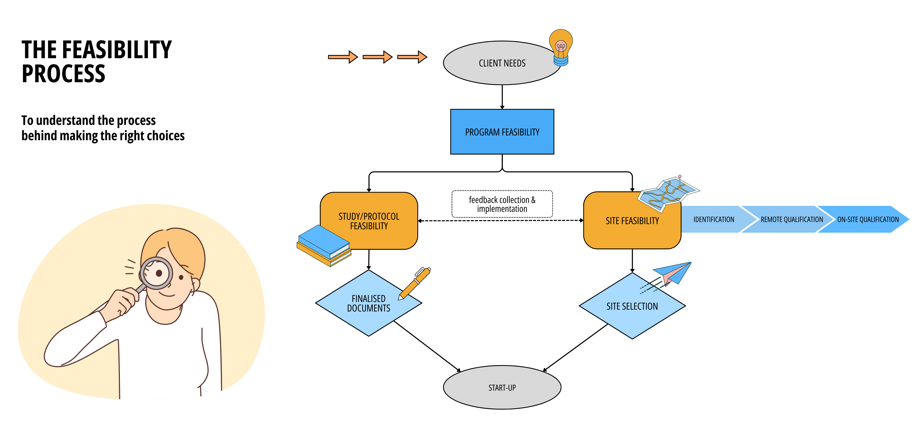
The BeEasy feasibility process is a comprehensive, multi-dimensional review that encompasses 3 key stages within the clinical study process:
Program feasibility
Study feasibility
Site feasibility
This process involves analyzing the study design, timelines, recruitment potential, regulatory requirements, and logistical considerations to identify and address potential obstacles that could hinder progress. By proactively resolving these challenges, sponsors and researchers can allocate resources to high-impact projects while avoiding costly delays or setbacks.
In the realm of clinical studies, thorough preparation and strategic foresight serve as the cornerstones of success. Central to this preparation is the vital process of feasibility assessment, which evaluates the practicality, resources, and structure within the scope of the entire project. Feasibility is the backbone of any successful project - it identifies hurdles, fine-tunes strategies, and transforms plans into actionable pathways for impactful results.
The BeEasy feasibility process is a comprehensive, multi-dimensional review that encompasses 3 key stages within the clinical study process:
Program feasibility
Study feasibility
Site feasibility
This process involves analyzing the study design, timelines, recruitment potential, regulatory requirements, and logistical considerations to identify and address potential obstacles that could hinder progress. By proactively resolving these challenges, sponsors and researchers can allocate resources to high-impact projects while avoiding costly delays or setbacks.
WHY IS FEASIBILITY IMPORTANT?
Major pitfalls of clinical studies stemming from poor feasibility include:
Instead, a well-conducted feasibility assessment provides substantial benefits, including:
study design alignment with patients' availability, sites' resources and study objectives
higher compliance to study plans in terms of timelines, budget & scope
adherence to regulatory and logistical requirements for clinical studies
increase in study credibility and trust by the scientific & regulatory community
Patient Recruitment Challenges: insufficient analysis of patient availability can lead to difficulties in enrolling enough eligible participants, delaying the study or compromising its statistical power. Underestimated Timelines: unrealistic timelines often result from inadequate feasibility assessments, causing significant delays in study execution and data collection. Resource Misallocation: inefficient planning of resources - such as staff, equipment, or facilities - may lead to operational bottlenecks and increased costs. Regulatory Setbacks: failure to account for local and international regulatory requirements can result in non-compliance, delaying approvals or halting the study altogether. Site Selection Issues: choosing sites without proper feasibility evaluation can lead to operational inefficiencies, such as low patient enrollment or logistical challenges. Nearly one-third of sites performing clinical studies never enroll a single patient. Budget Overruns: underestimating the financial requirements of the study due to a lack of feasibility evaluation may lead to unexpected expenses and funding shortfalls. Logistical Barriers: poor feasibility assessment can overlook logistical complexities, including transportation of study materials or availability of specialized equipment.

EXPERIENCE IN ACTION
See the data that sets us apart.
INTERESTED IN OUR SERVICES? LET’S TALK.
CONNECT
WITH BEEASY
Contact us here or check us out on our LinkedIn.
Get in touch today and discover how we can support your next steps.



